Michael Addition Reactions
(Kinetics of Conjugated Additons)
Michael addition reactions have become a popular polymerization method in recent years. This
type of reaction meets many of the requirements of a click reaction including no by-products, orthogonal, fast,
and high yield at low reaction temperatures.1
Michael addition typically refers to the base catalyzed addition of an
activated nucleophile (Michael donor) to electrophile (Michael acceptor). Very popular Michael addition systems are electron deficient C=C acceptor bonds as present in acrylates and acidic C-H donor bonds as present in acetoacetates and malonates.2 This type of reaction is
typically catalyzed by a Lewis base such as an enolate anion yielding
a nucleophilic carbanion that can quickly add to an acrylic double bond. The suggested reaction mechanism between acetoacetates and acryloyl compounds is shown below.3
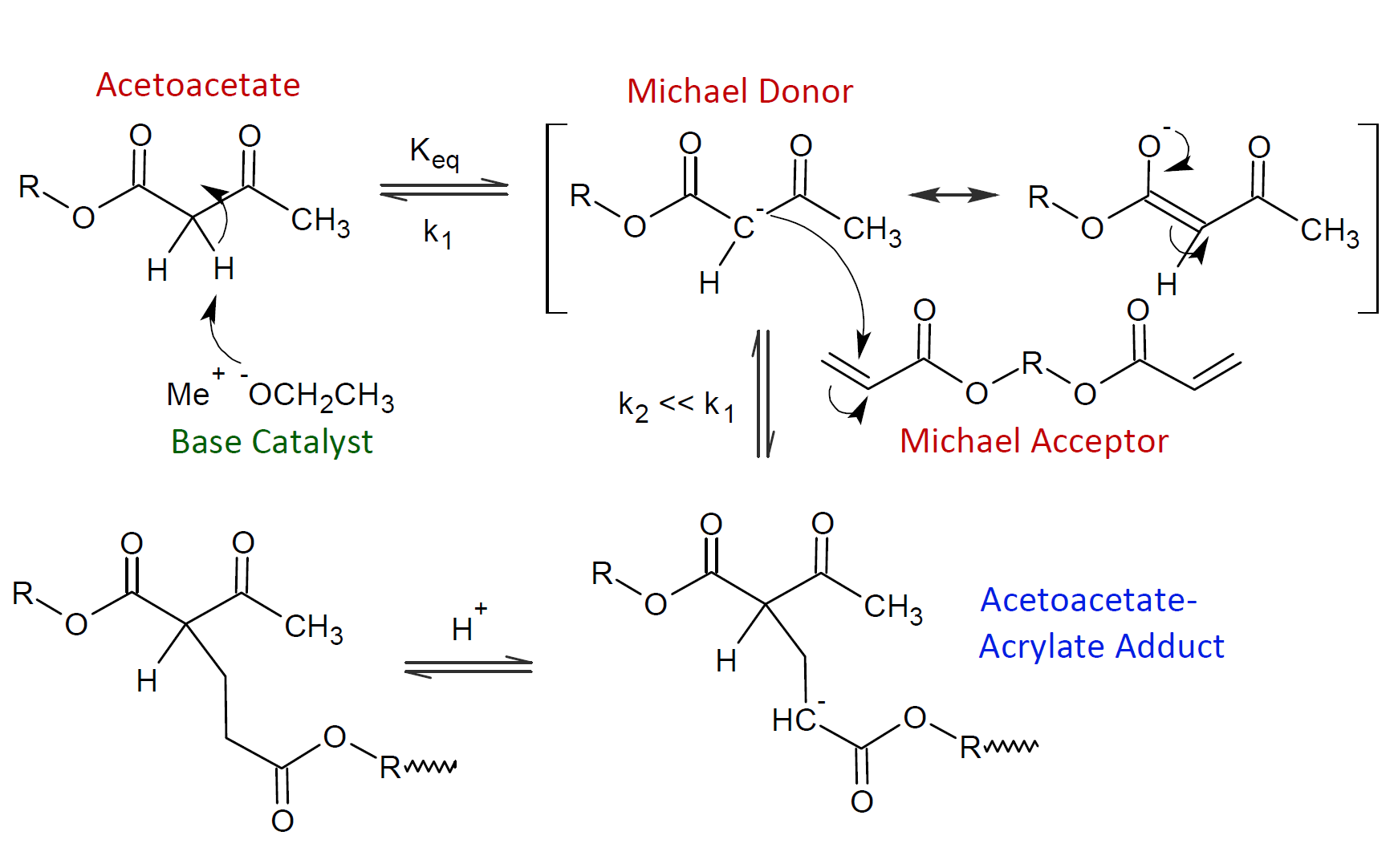
This reaction requires a Lewis base strong enough to abstract a proton from the acidic C-H bond (Michael donor) which has to be a relative strong Lewis acid itself. The pKa of an acetoacetate C-H is around 12 and of a malonate it is even higher (> 13).2,3 Thus, Michael addition of the carbanions of these two compounds to acrylates proceeds quickly. The reaction, however, takes only place in the absence of acidic compounds since these will react with the base catalyst rendering it ineffective for hydrogen abstraction.
The acetoacetate-acrylate product has a second acidic hydrogen which can also undergo a Michael addition to another double bond. The second hydrogen, however, has a much larger pKa of about 13.3,4 As a consequence, the concentration of the first Michael adduct will be low since it quickly reacts with another acrylate molecule as shown below.3
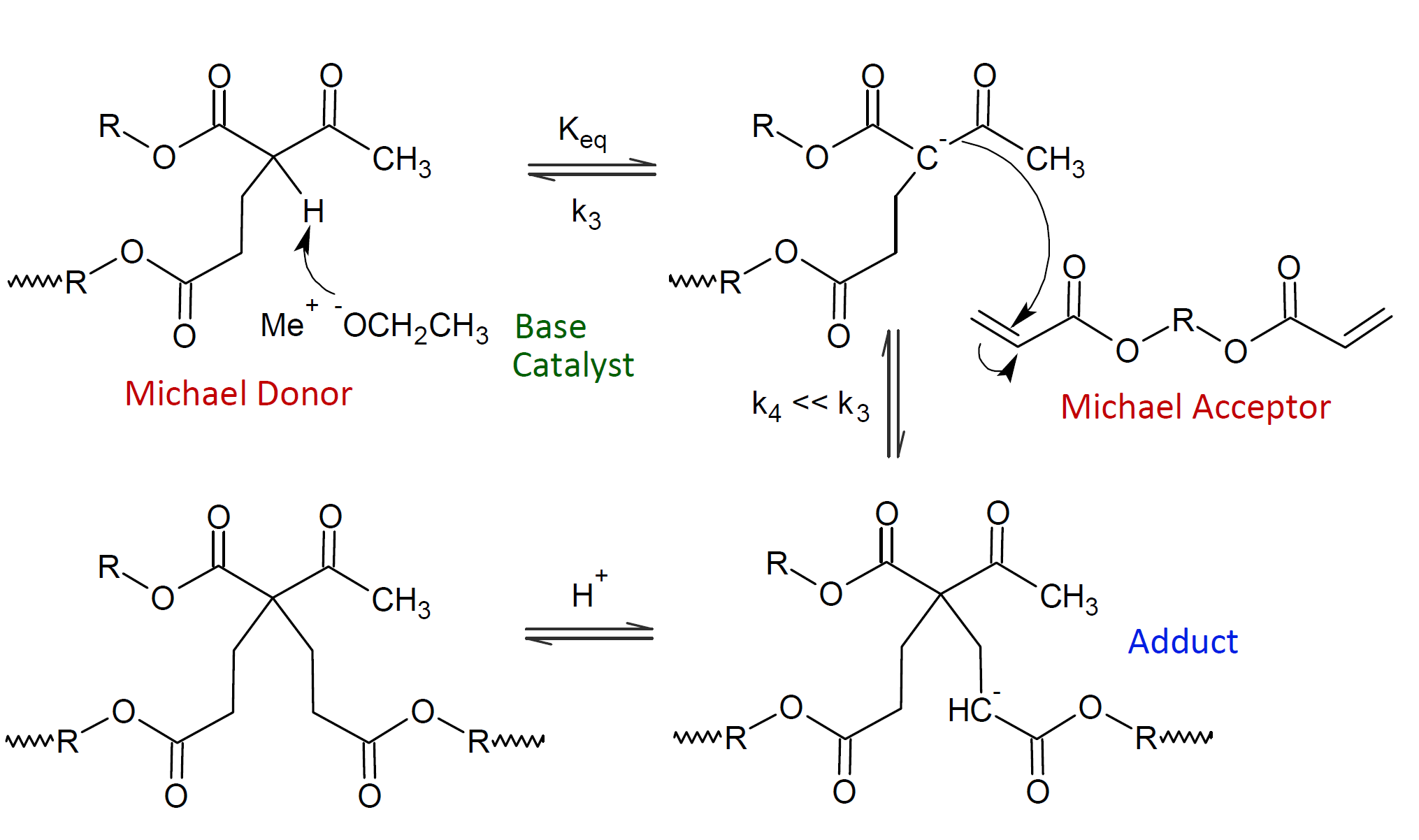
Mather et. al. assumed that the concentration of enolate anions at any stage is not strongly affected by the second addition reaction.3 Then a simple rate law applies; the rate-determining step is the addition of an acetylacetonate anion to an acrylate in the reaction sequence.5 For this case, the rate of addition is given by:3
R = k2 [AcAc-] [Acrylate]
The concentration of acetylacetonate anions, [AcAc-], can be calculated if the equillibrium constant of the deprotonation, Keq is known:
Keq = [AcAc-] [BH+] / [AcAc] [B] ⇒
[AcAc-] = Keq [AcAc] [B] /
[BH+]
Substitution of this equation into the rate equation yields
R = k2 Keq [Acrylate] [AcAc] [B] / [BH+]
Thus the type of base catalyst has a significant effect on the reaction rate. For strong bases, most of the base is protonated and the concentration of the enolate anion is approximately equal to that of the base at steady state. Then the reaction rate has a pseudo first-order dependence on acrylate concentration:3
Rexp ≈ kexp [Acrylate]
where the observed rate constant kexp is a function of the base concentration, [B].
References & Notes
-
G. Gonzalez, X. Fernandez-Francos, A. Serra, M. Sangermanoc and X. Ramis, Polym.
Chem., 6, 6987 (2015) -
R. Brinkhuis, J. Schutyser, F. Thys, E. De Wolf, T. Buser, J. Kalis, N. Mangnus, F. Van Wijk, Europ. Coat. J., 5, 34 - 40 (2015)
B.D. Mather, K. Viswanathan, K.M. Miller, T.E. Long, Prog. Polym. Sci. 31, 487–531 (2006)
-
R.J. Clemens, F.D. Rector, J. Coat. Technol., 61, 83-91 (1989)
J.A. Markisz and J.D. Gettler, Canadian Journal of Chemistry, 47, 1965 (1969)
-
D. Herova, P. Pazdera, Monatshefte fuer Chemie, pp. 1-20, Dec. 2014
February 28, 2020